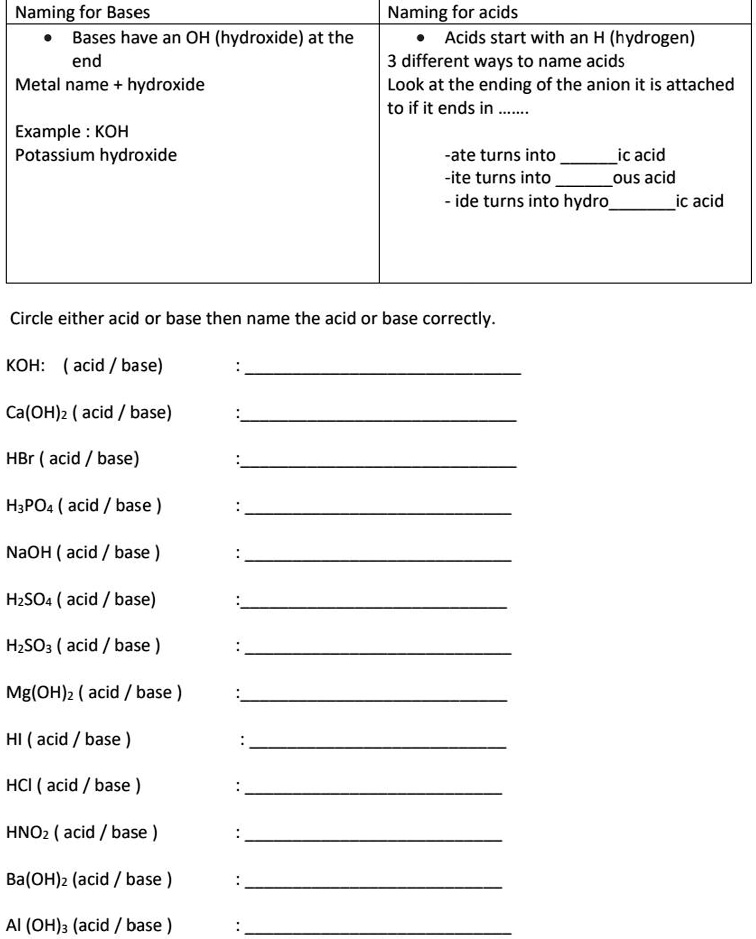
SOLVED: Naming for Bases Bases have an OH (hydroxide) at the end Metal name hydroxide Naming for acids Acids start with an H (hydrogen) 3 different ways to name acids Look at

Acid Base Definitions Originally recognized by properties like taste, feel, reactions with indicators – Acids taste sour and turn blue litmus red – Bases. - ppt download

The Chemistry of Acids and Bases Chapter Strong and Weak Acids/Bases Acids and bases into STRONG or WEAK ones.Acids and bases into STRONG or WEAK. - ppt download
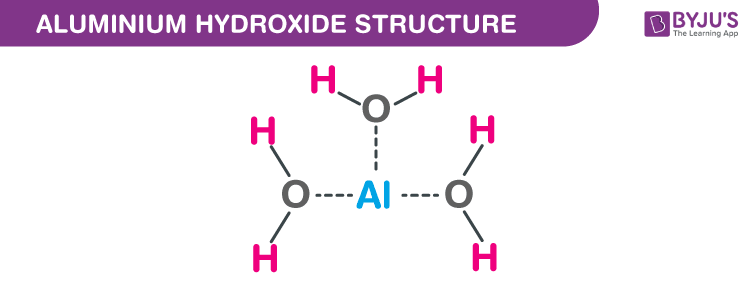
Aluminium Hydroxide (Al(OH)<sub>3</sub>) - Structure, Molecular mass, Properties, Uses and FAQs of Aluminium Hydroxide

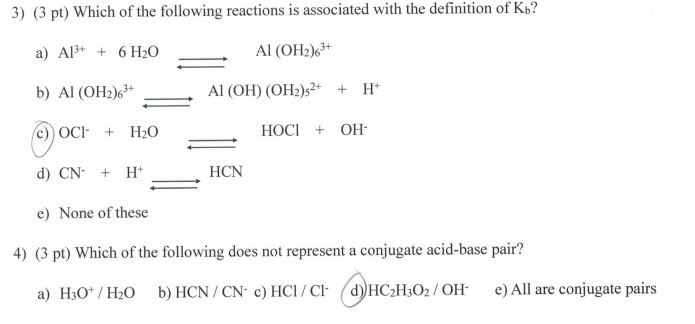

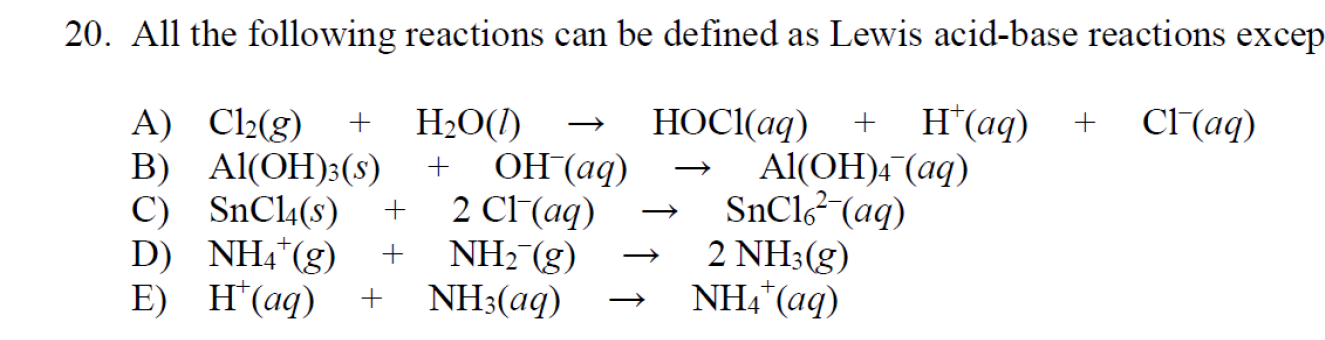







![Conjugate base of [Al(H(2)O)(6)]^(3+) is Conjugate base of [Al(H(2)O)(6)]^(3+) is](https://d10lpgp6xz60nq.cloudfront.net/ss/web/561545.jpg)
