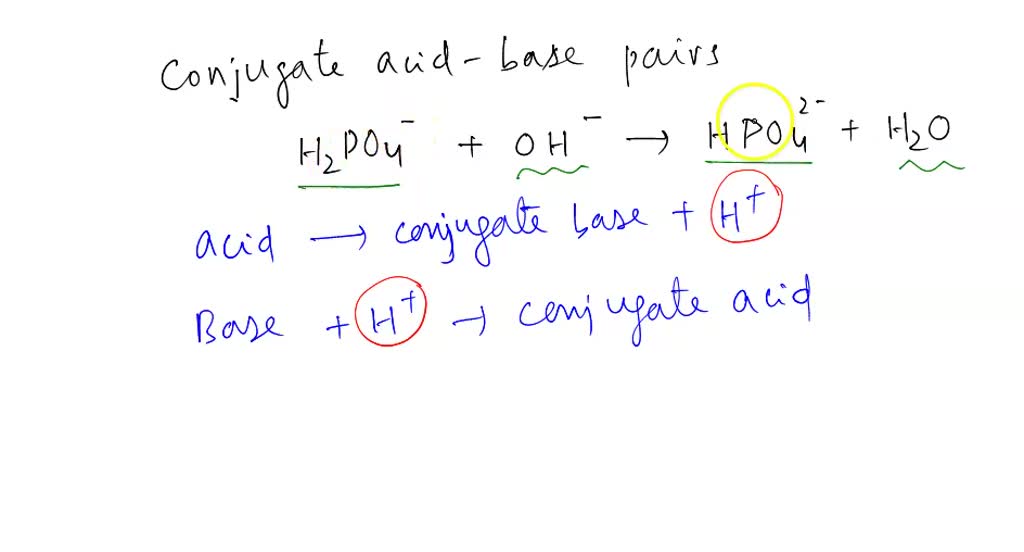
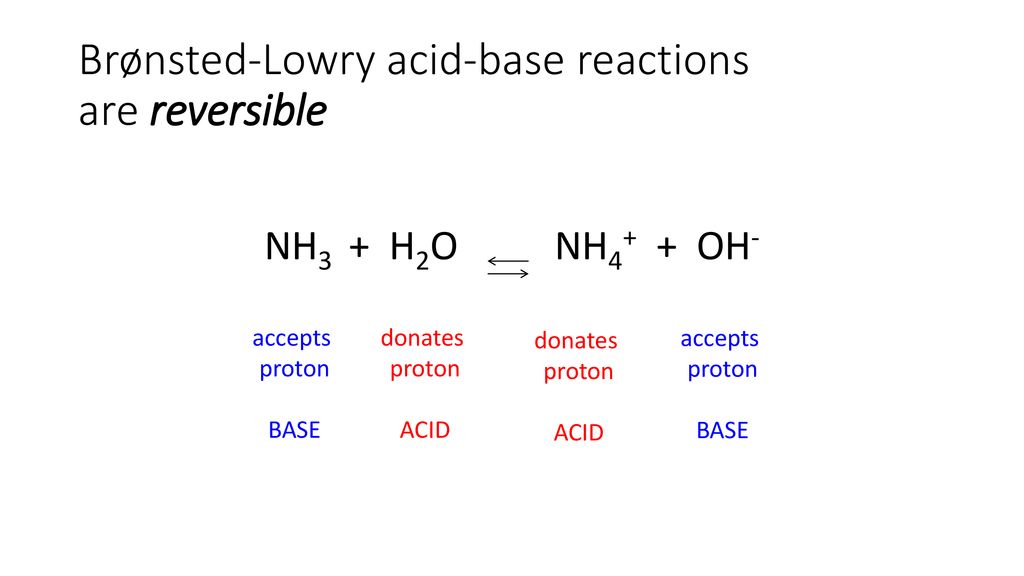
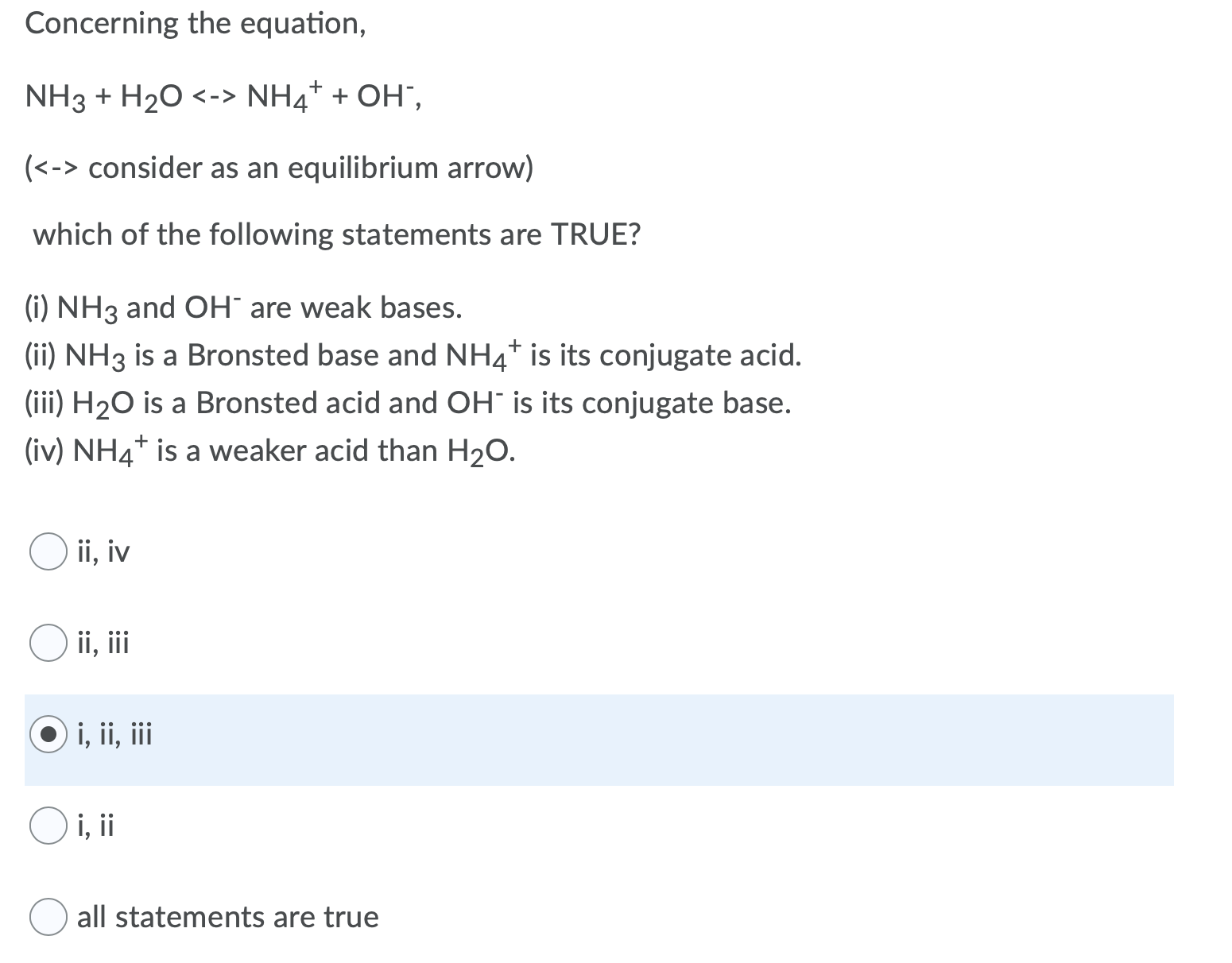
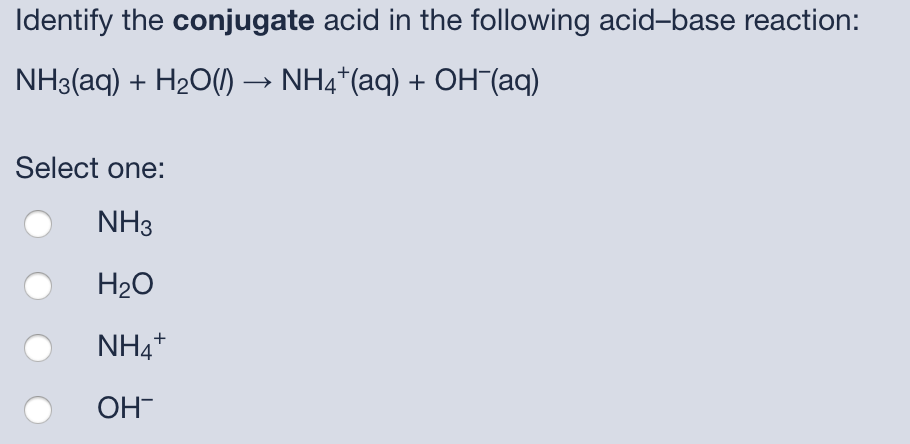
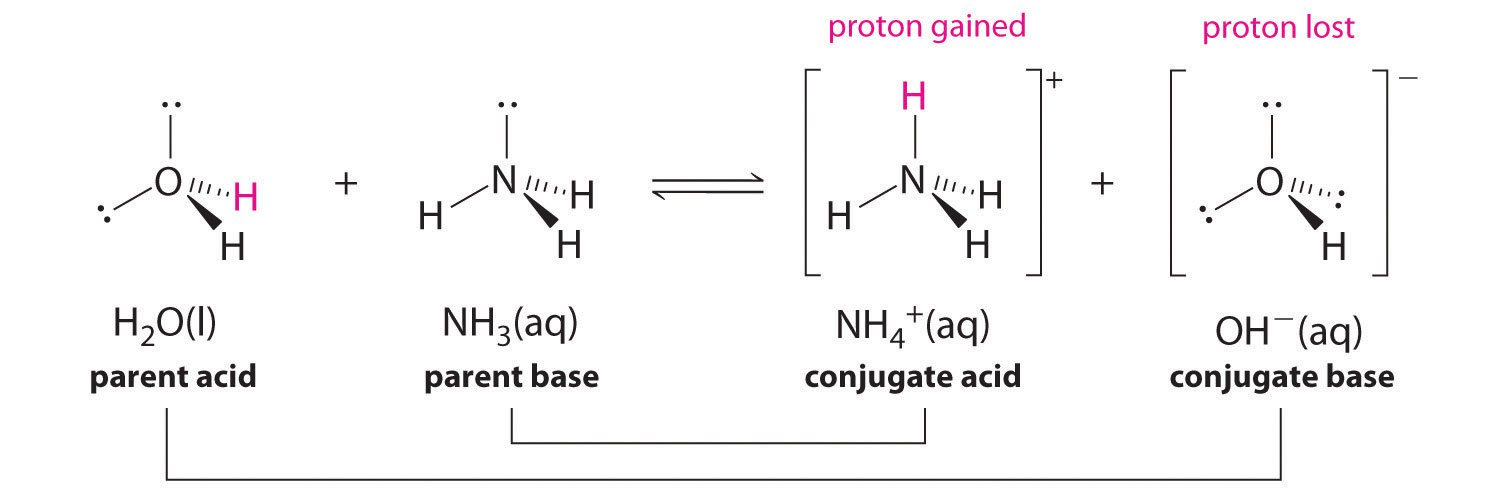
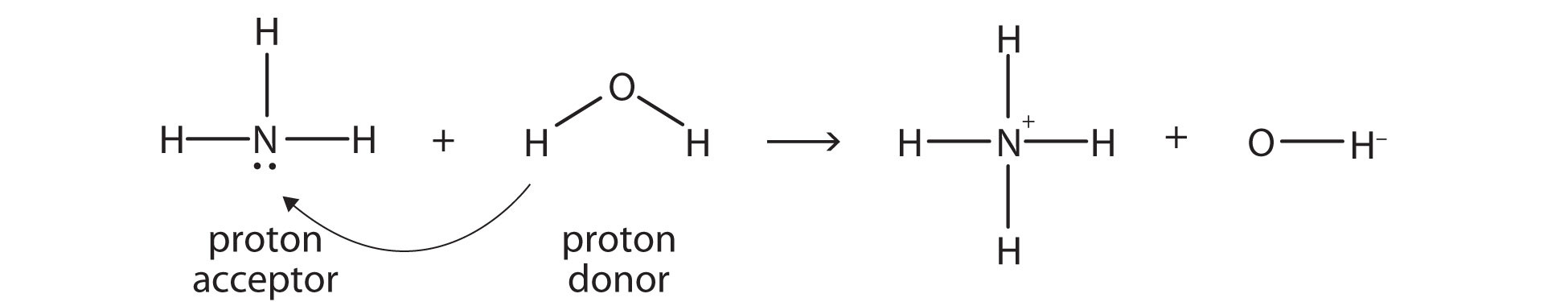
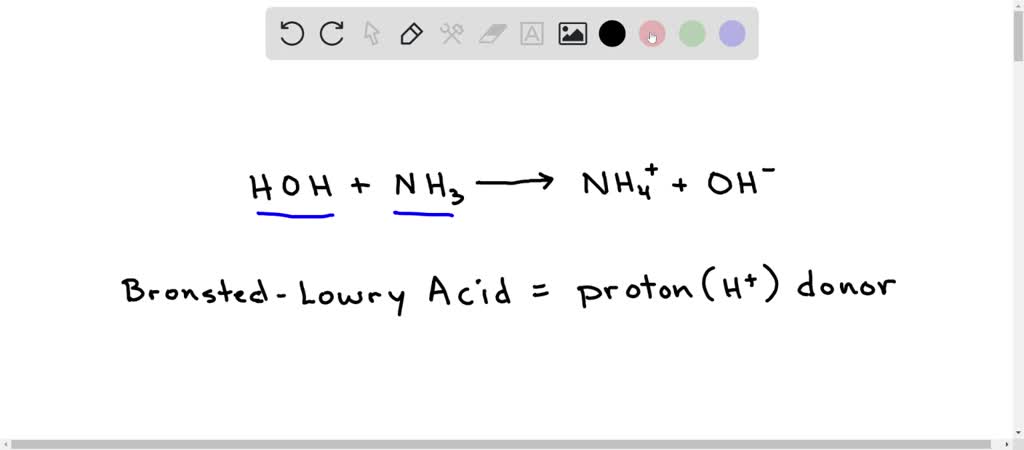
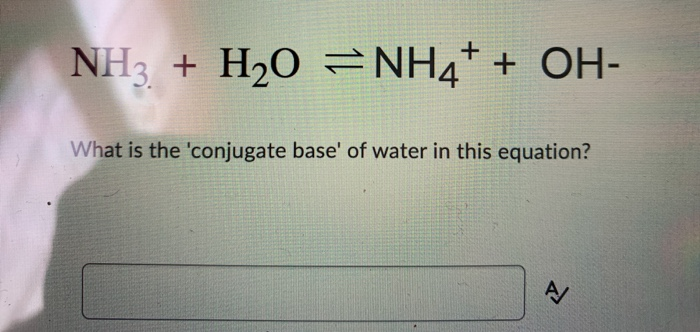An acid-base reaction can occur when ammonia (NH3) and water (H2O) are mixed. Draw the curved arrows depicting the electron flow for the following acid- base reaction. Draw the conjugate acid and

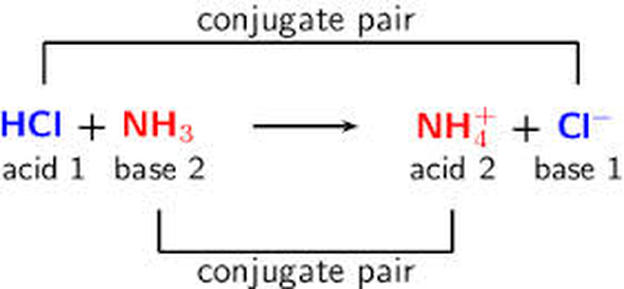
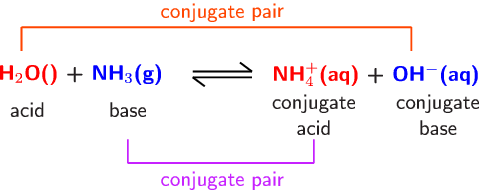
SOLVED: In the reaction NH3 (aq) + H2O (I) <–> NH4+ (aq) + OH- (aq), which is the conjugate acid-base pair? OH- NH3 NHA+, OH- NH3, H2O NH4+, NH3
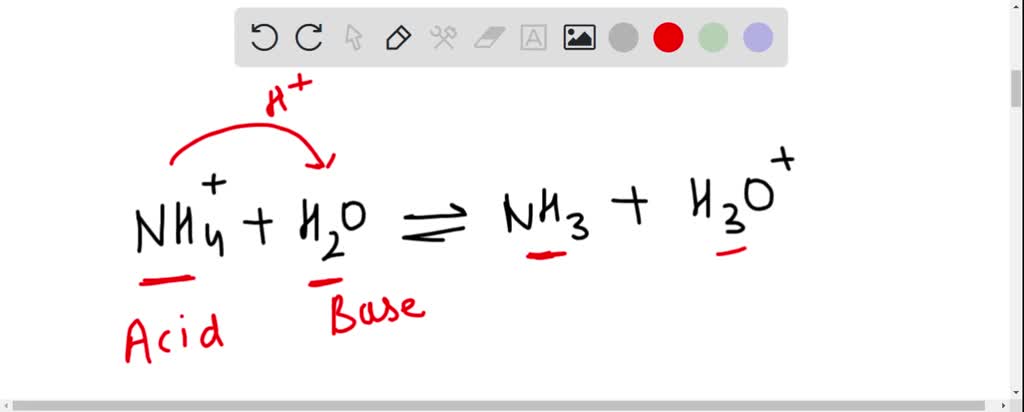
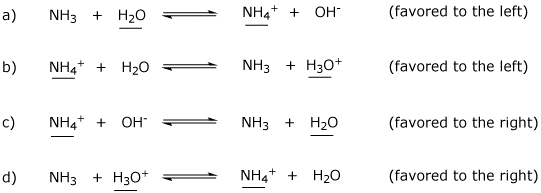
SOLVED: In the following reaction: NH4+ + H2O = NH3 + H3O+ A) H2O is a base and NH3 is its conjugate acid B) NH4+ is an acid and H20 is its

OneClass: Questions 3 and 4. Consider the reaction shown below: NH2-(ag) + H2O(l) ê·¼ NH3(gg) + OH-(a...