Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa
Why is barium hydroxide considered aqueous when barium fits into the 'most' category on a solubility chart? - Quora

A. Calculate the pH of a 0.10 M solution of barium hydroxide, Ba(OH)2 - Home Work Help - Learn CBSE Forum

SOLVED: Beryllium oxide is what we call amphoteric-it can react with both acids and bases. BeO 2HzO+ Hzo [Be(HzO)4J2+(aq) BeO 2OH- HzO [Be(OH)4J2 (aq) Explain how various acid-base definition can be applied

Question Video: Using Strong Acid-Strong Base Titration Data to Calculate the Concentration of the Base | Nagwa

Determination of chemical properties of meat: Determination of volatile basic nitrogen (VB-N), trimethylamine oxide nitrogen (TMAO-N) and trimethylamine-nitrogen (TMA-N) by Conway's micro-diffusion method (N/150 hydrochloric acid and N/70 barium hydroxide)

Acids and Bases For now, acids ionize in aqueous solutions to form a hydrogen ion (H + ). -- “proton donors” -- monoprotic acids e.g., -- diprotic acids. - ppt download
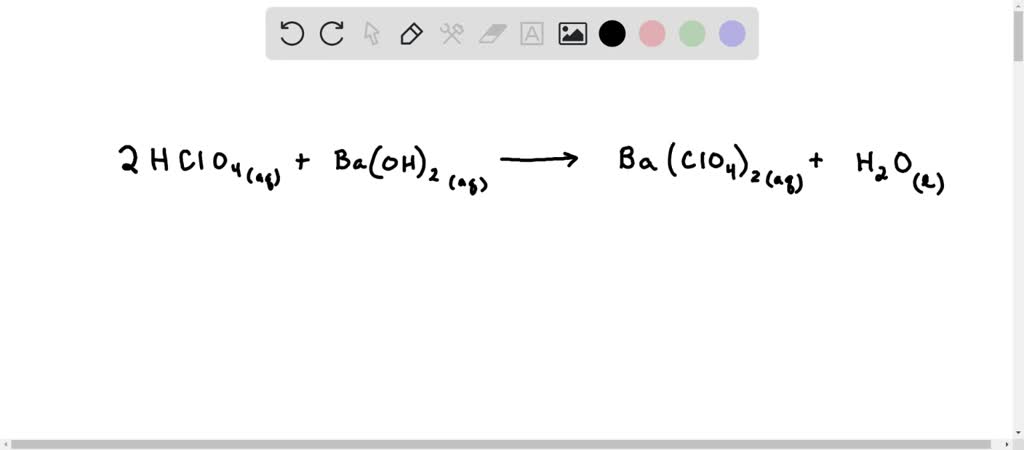
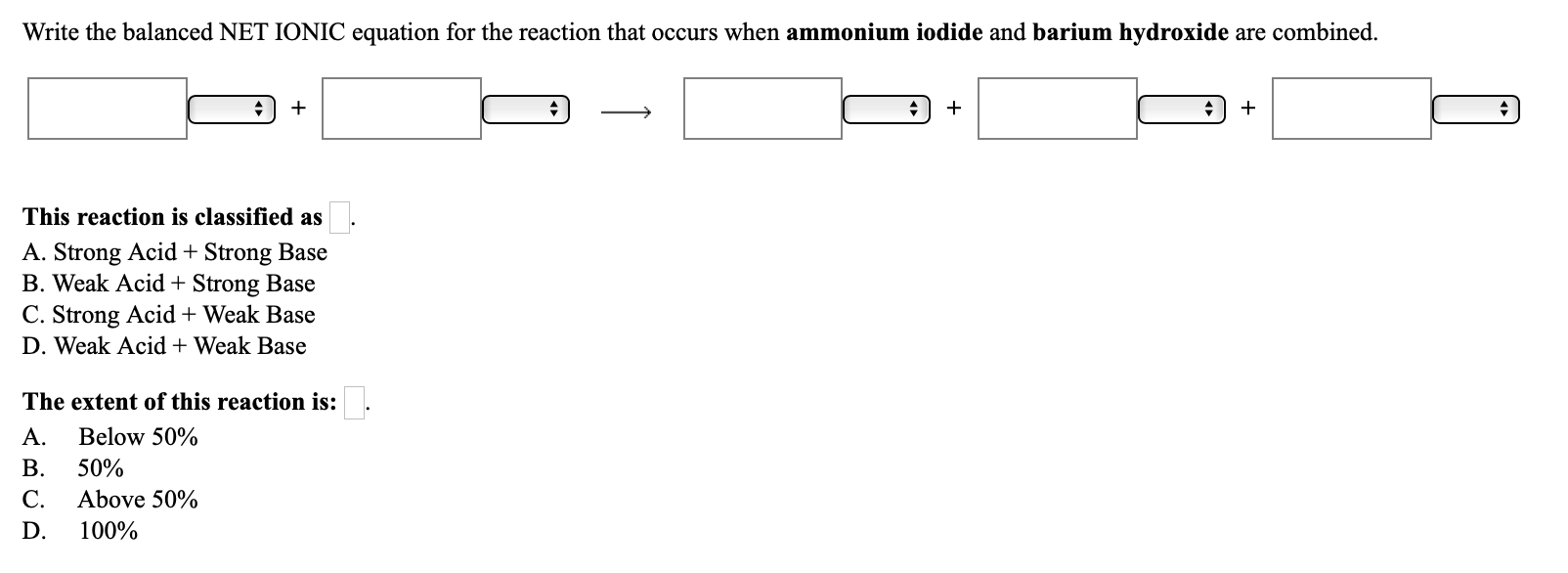
SOLVED: 22. The spectator ions in the reaction between aqueous perchloric acid and aqueous barium hydroxide are: OH- and ClO4- H+, OH-, ClO4- and Ba+2 H+ and OH- H+ and Ba+2 ClO4-

25 ml of a solution of barium hydroxide on the titration with a 0.1 molar solution of hydrochloric acid gave a titre value of 35 ml. The molarity of barium hydroxide solution is:

ACIDS & BASES module i.An acid is a chemical substance that …………………in water to produce ………………. ions. ii.A base is a chemical substance that ………………in. - ppt download