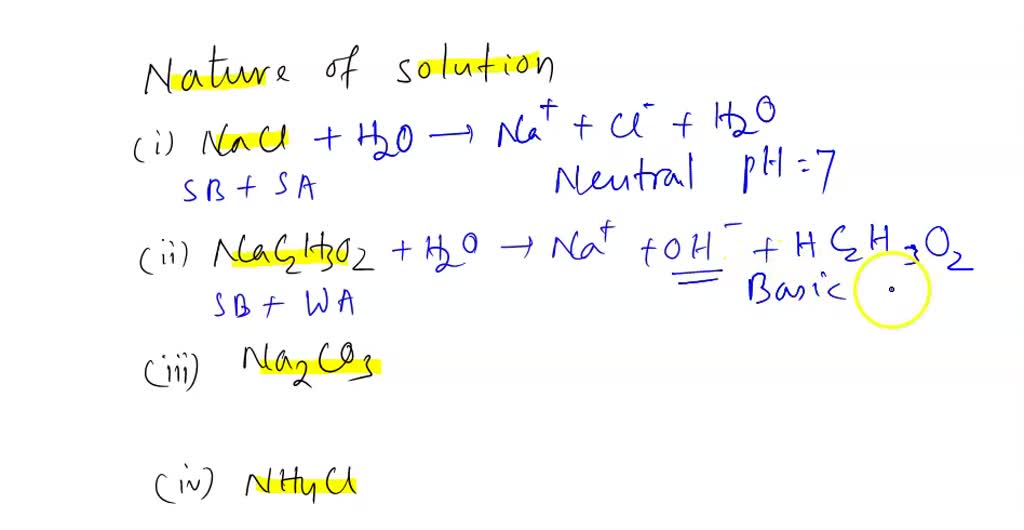
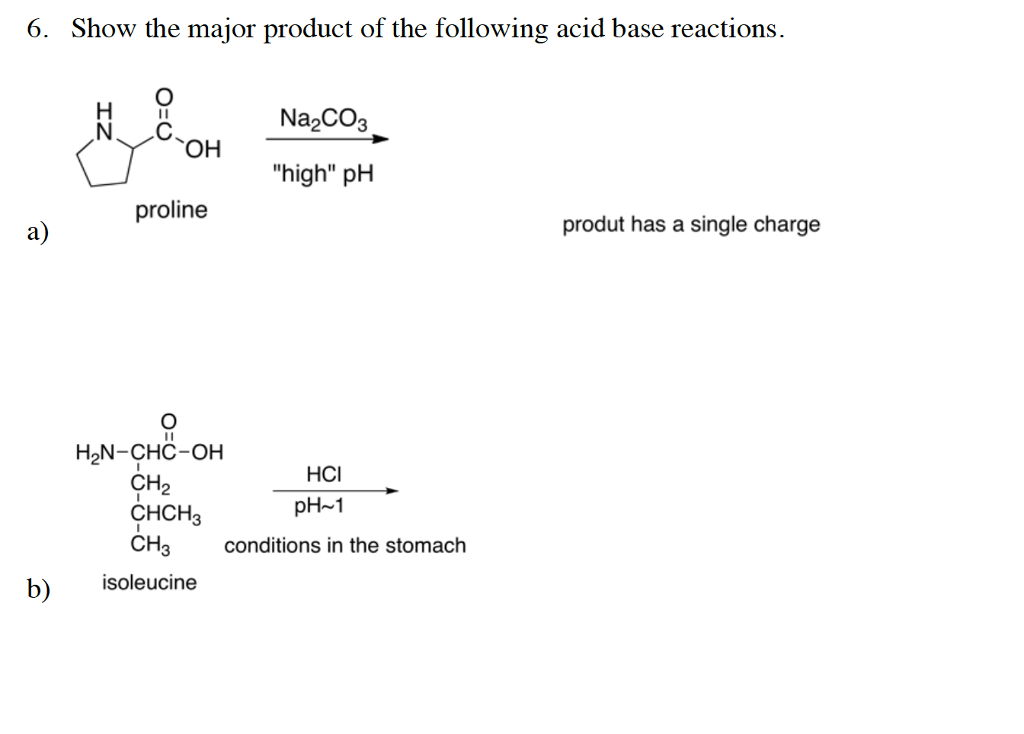
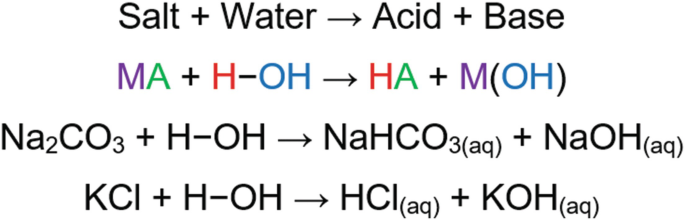
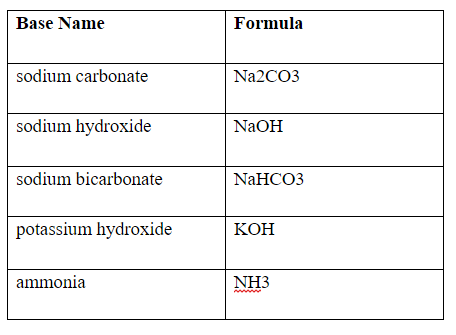
Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

In a buffer solution consisting of a weak acid and its salt, the ratio of the concentration of salt to acid is increased tenfold, then the pH of the solution will:

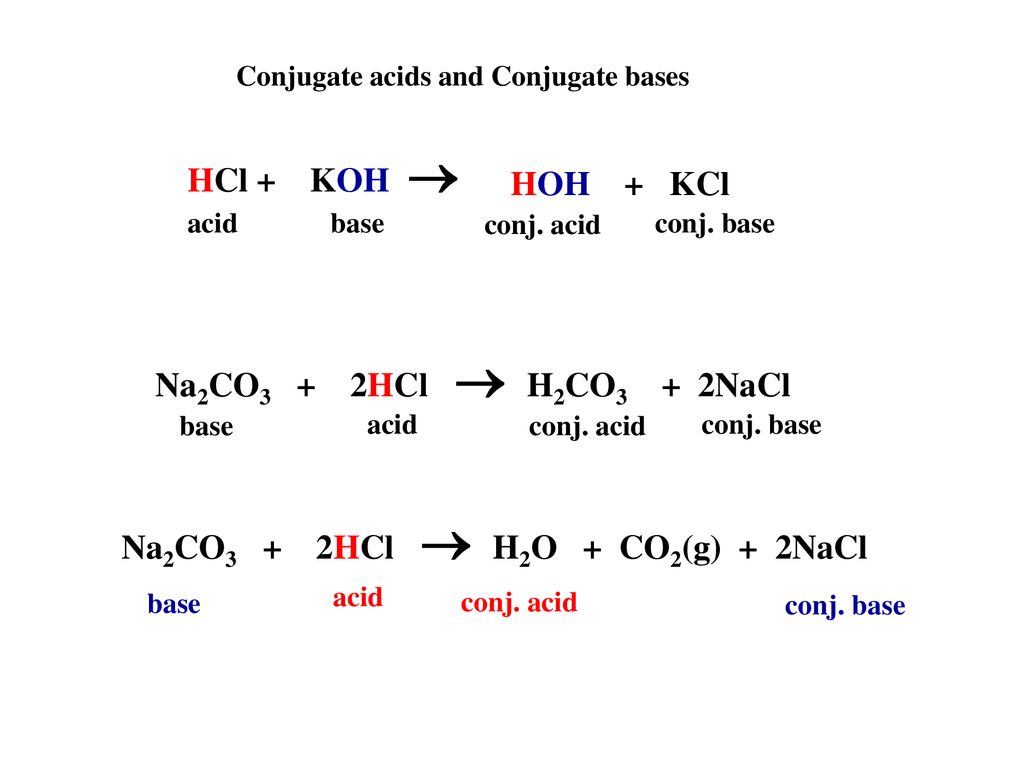
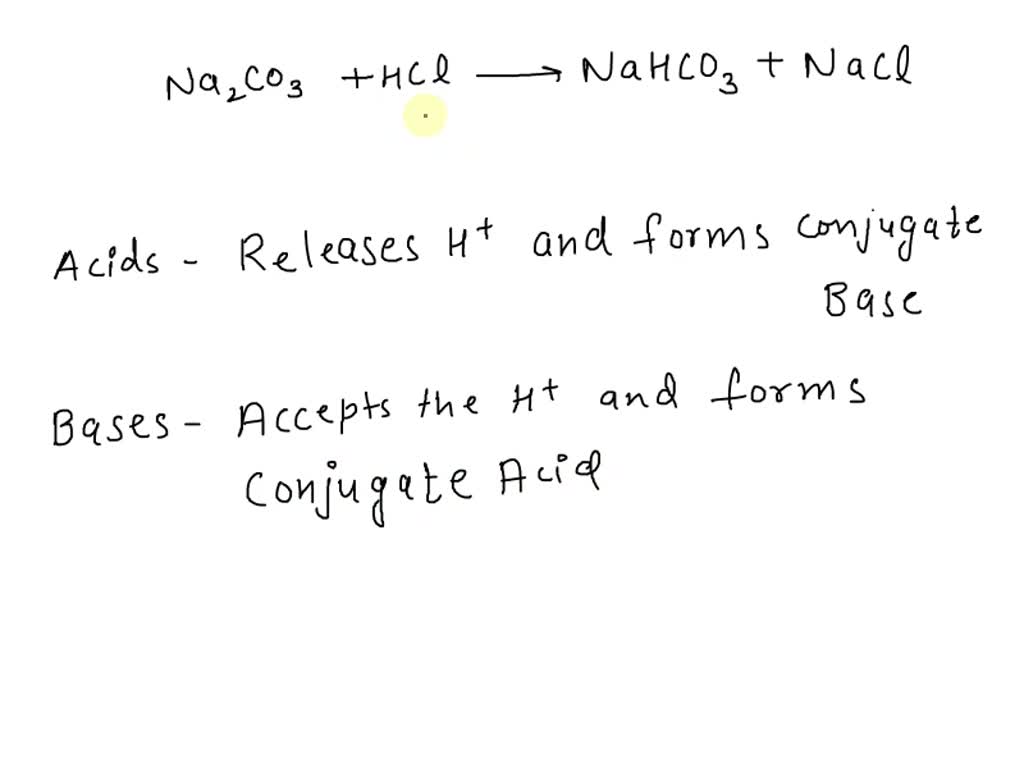
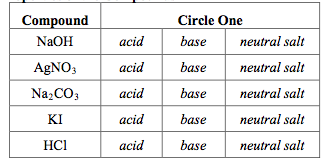
Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid | Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid Hello, Chemistry Enthusiasts! For today's
What is going on in the formula 2NaOH+ CO2 → Na2CO3+ H2O? What is the type of reaction? How are the bases and acids reacting with this product? What is created, and

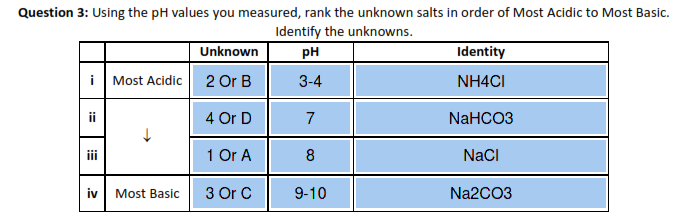
The titration of Na2CO3 with HCl has the following qualitative profile: a. Identify the major species in solution as points A-F. b. For the titration of 25.00 mL of 0.100 M Na2CO3

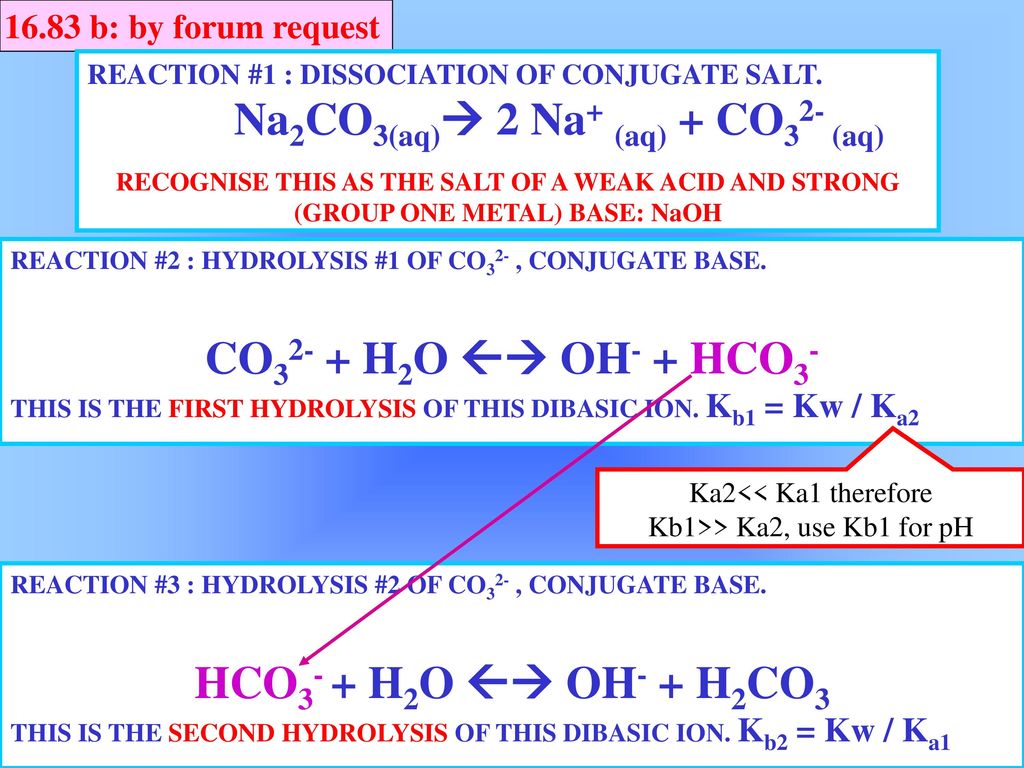
physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange